DAY 1
11.5.20
6th period.
Good Morning Boys,
Today we will revise the rules of Nomenclature of organic compounds.
LEARNING OUTCOMES :
Students will be able to






11.5.20
6th period.
Good Morning Boys,
Today we will revise the rules of Nomenclature of organic compounds.
LEARNING OUTCOMES :
Students will be able to
- Identify Functional group
- use the rules to nomenclate the organic compound.
- correlate with the terms used.
Rule I
Longest chain rule The chain containing the principal functional group, secondary functional group and multiple bonds as many as possible is the longest possible chain.
In the absence of functional group, secondary group and multiple bonds, the chain containing the maximum number of C-atoms will be the longest possible chain.
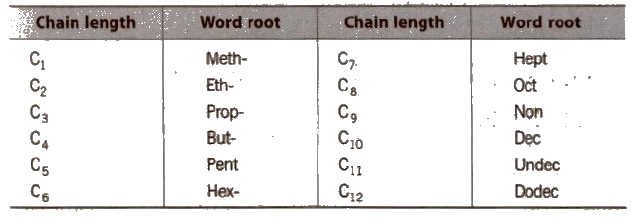
Word Root for Carbon Chain

Rule 2
Lowest number rule Numbering is done in such a way so that
1. branching if present gets the lowest number.
2. the sum of numbers of side chain is lowest.
3. principal functional group gets the lowest number.
2. the sum of numbers of side chain is lowest.
3. principal functional group gets the lowest number.
Select the principal functional group from the preference series :

Functional group other than the principal functional group are called substituents.
Rule 3.
Naming the prefixes and suffixes Prefix represents the substituent and suffix is used for principal functional group.
Primary prefixes are cycle, bicycle, di, tri etc
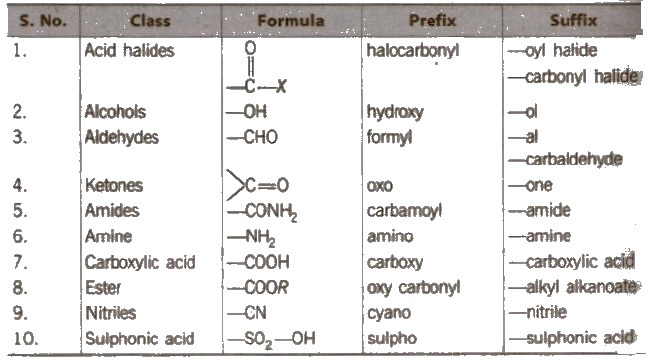
Secondary prefixes are tabulated below :

Primary suffix are ene, ane, or yne used for double, single and triple bonds respectively.
Secondary suffixes are tabulated below :

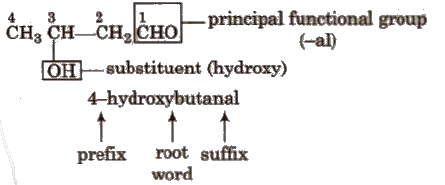
Hence. according to the rules. given above, the IUPAC name of a compound can be written as
Prefixes + Root word + Suffixes Primary prefix + secondary prefix + Root word + primary suffix + secondary suffix

Questions :
Give the structural formula for each of the following :
Give the structural formula for each of the following :
Octane, 3-ethylpentane, hex-1-ene, 6-methylhept-3-yne
Give the IUPAC name for the following compound:




THATS ALL FOR THE DAY.
MARK YOUR ATTENDANCE ON THE FORM.
Utkrist Gupta
ReplyDelete11-D
Present
Tanmay Jain
ReplyDelete11-D
Present
Good morning ma'am
ReplyDeleteSarvesh Kumar 11-D
Present
Good morning ma'am, Yumn jame 11th D present.
ReplyDeleteSiddharth karnish present for 6th period
ReplyDeleteGood morning ma'am
ReplyDeleteYashas Yadav 11-D
Present
Tathastu Bagchi 11D present
ReplyDeleteRatnango Ghosh
ReplyDelete11 D