DAY 1 6th period
21.4.20
Good Afternoon Boys,
Learning Objective :
students will be able to
- Define Mole
- Apply Mole concept to numericals.
Notes and numerical have to be copied in your notecopy.Mole
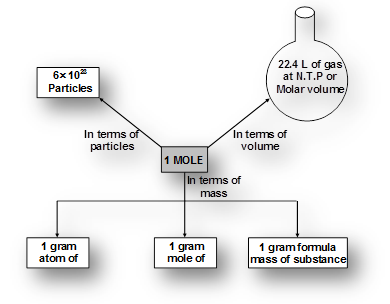
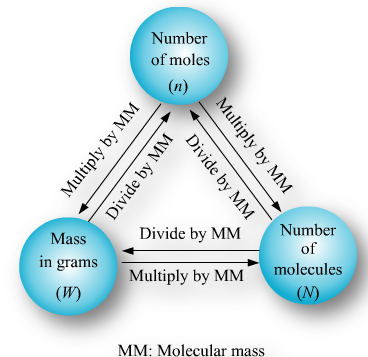
It is defined as a unit which represents 6.023 x1023 particles of same matter.A mole (symbol mol) is defined as the amount of substance that contains as many atoms, molecules, ions, electrons or any other elementary entities as there are carbon atoms in exactly 12 gm of carbon
Solution:
- 1 mole of Ag atoms = 108 g
= 6.022 x 1023 atoms
6.022 x 1023 atoms of silver have mass = 108g
Mass of one atom of silver

- 1 mole of CO2 = 44 g
= 6.022 x 1023 molecules
Thus, 6.022 x 1023 molecules of CO2 has mass = 44 g
1 molecule of CO2 has mass = 7.307 ´x 10-23 g
______________________________________________________________
Question 2: Calculate the number of molecules present
- in 34.20 grams of cane sugar (C12H22O11)
- in one litre of water assuming that the density of water is 1 g/cm3
- in one drop of water having mass 0.05 g.
Solution:
- 1 mole of C12H22O11 = 342 g
= 12 x 12 + 22 x 1 + 11 x 16 = 342 amu
= 6.022 x 1023 molecules
Now 342 g of cane sugar contain 6.022 x 1023 molecules.
34.2 g of cane sugar will contain
= 6.022 x 1022 molecules
- 1 mole of water = 18 g = 6.022 x 1023 molecules.
- Mass of 1 litre of water = Volume x density = 1000 x 1 = 1000 g
Now 18 g of water contains = 6.022 x 1023 molecules.
1000 g of water will contain =  3.346 x 1025 molecules
3.346 x 1025 molecules
 3.346 x 1025 molecules
3.346 x 1025 molecules- 1 mole of H2O = 18 g = 6.022 x 1023 molecules.
Mass of 1 drop of water = 0.05 g
Now 18 g of H2O contain = 6.022 x 1023 molecules.
0.05 g of H2O will contain =  0.016 x 1023 molecules.
0.016 x 1023 molecules.
 0.016 x 1023 molecules.
0.016 x 1023 molecules.
________________________________________________
Question 3: Calculate the number of moles in each of the following:
- 392 grams of sulphuric acid
- 44.8 litres of carbon dioxide at STP
- 6.022 x 1023 molecules of oxygen
- 9.0 grams of aluminium
- 1 metric ton of iron (1 metric ton = 103 kg )
- 7.9 mg of Ca
- 1 mole of H2SO4 = 98 g
Thus 98 g of H2SO4 = 1 mole of H2SO4
392 g of H2SO4 =  = 4 moles of H2SO4
= 4 moles of H2SO4
 = 4 moles of H2SO4
= 4 moles of H2SO4- 1 mole of CO2 = 22.4 litres at STP
i.e. 22.4 litres of CO2 at STP = 1 mole
44.8 litres of CO2 at STP = 2 moles CO2
- 1 mole of O2 molecules = 6.022 x 1023 molecules.
6.022 x 1023 molecules = 1 mole of oxygen molecules.
- 1 mole of Al = 27 g of Al
9 g of aluminium = 0.33 mole of Al
0.33 mole of Al
 0.33 mole of Al
0.33 mole of Al- 1 metric ton of Fe = 103 kg = 103000g
1 mole of Fe = 56 g of Fe
103000 g of Fe = 1.839 x 102 moles
Stay safe
Take care
FOR MARKING YOUR ATTENDANCE
AND CLICK SUBMIT.

TYPE YOUR NAME AS PER THE DIRECTIVES GIVEN BELOW ⇓

.
 x 10-3 g of Ca
x 10-3 g of Ca
Utkrist Gupta
ReplyDelete11-D
present
Tanuj pant
ReplyDelete11D
Present
Anshuman Jaison of 11D is present
ReplyDeleteLakshya Gunjan
ReplyDelete11-D
srivatsa davuluri 11d
ReplyDeleteOYSTER DCOSTA 11 D PRESENT
ReplyDeleteKrrishvarshney 11d present
ReplyDeleteAarmaan chhibber,11-D present
ReplyDeleteRISHIT GUPTA ,11-D PRESENT
ReplyDeleteYumn Jame 11th D present
ReplyDeleteGood morning maam
ReplyDeleteTeghveer Singh class 11-D
PRESENT
ReplyDeleteSamuel DM 11-D
Present
Good Morning Ma'am
ReplyDeleteSoham Kulkarni 11-D
Attendance form submitted
Vatsal Aggarwal 11D is present
ReplyDeleteShambhava S. 11-D -: I've submitted the attendance form, ma'am.
ReplyDeleteJaskeerat Singh (11-D) - Present
ReplyDeleteTuhin Raha
ReplyDelete11-D
present
Tanmay Jain
ReplyDelete11-D
Present
Sarvesh Kumar
ReplyDelete11-D is PRESENT
ADITYA VOHRA 11-D present
ReplyDeletegood morning ma'am
ReplyDeletesami ansari 11-D
present
Good morning ma'am
ReplyDeleteAvikam Gupta
11-D
Present
Tathastu Bagchi 11D present
ReplyDeleteGood Morning,
ReplyDeleteThis is Joseph James Nedumpara of XI D
UTSAV RAJORA PRESENT 11D
ReplyDeleteRatnango Ghosh
ReplyDelete11 D Present
Shreyas Aditya baksi
ReplyDelete11 d present mam
Siddharth karnish 11-D
ReplyDelete(PRESENT)
good morning
ReplyDeleteBhumik Tandon of class 11th D is present
Good morning ma'am
ReplyDeleteRahul Bandhu
11-D
Good morning ma'am
ReplyDeleteYashas Yadav 11-D
Present
Aashish Parker XI-D Present
ReplyDeleteartham pedneker
ReplyDeleteattendance submitted through google forms
students you need to draw the mindmap given for mole concept
ReplyDelete